You are viewing the article: 6Na + N2 → 2Na3N |Traloitructuyen.com at Traloitructuyen.com
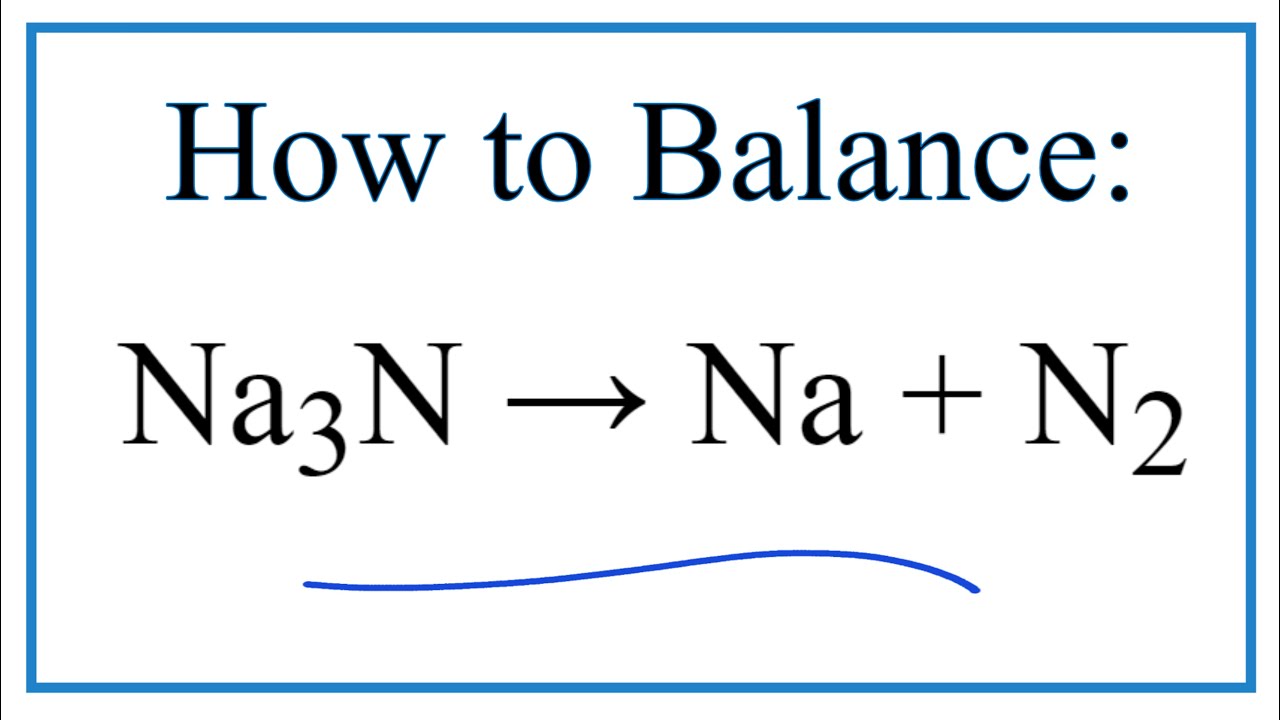
This is an oxidation-reduction (redox) reaction:
6 Na - 6 e- → 6 NaI (oxidation)
2 N + 6 e- → 2 N-III (reduction)
Na is a reducing agent, N2 is an oxidizing agent.
[external_link offset=2]
Reactants:
- Na
- Names: Sodium , Na , Element 11 , Natrium
- Appearance: Silvery solid in various forms
- N2
- Names: Dinitrogen , Nitrogen (compressed gas) , Nitrogen (liquified)
- Appearance: Odourless colourless compressed gas ; Odourless colourless extremely cold liquid
Products:
- Na3N
- Names: Sodium nitride
[external_footer]
Một số từ khóa tìm kiếm liên quan:
- N2 H2
- Na3N ra NH3
- N2 + O2
- Na N2
See more articles in the category: Hóa học

