Or you want a quick look: Aqueous Ammonia
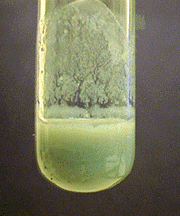
Ammonia reacts with chromium(III) ion to precipitate gray-green chromium(III) hydroxide: \[\ce{Cr^{3+}(aq) + 3NH3(aq) + 3H2O(l) <=> Cr(OH)3(s) + 3NH4^{+}(aq)}\] \(\ce{Cr(OH)3}\) dissolves only to a slight extent in excess ammonia. Boiling the solution causes the chromium(III) hydroxide to reprecipitate. Strong bases such as \(\ce{NaOH}\) also precipitate \(\ce{Cr(OH)3}\), but the precipitate dissolves in excess hydroxide. \[\ce{Cr^{3+}(aq) + 3OH^{-}(aq) <=> Cr(OH)3(s)}\] \[\ce{Cr(OH)3(s) + OH^{-}(aq) <=> Cr(OH)4^{-}(aq) (green) }\] In basic solution, hydrogen peroxide oxidizes \(\ce{Cr(III)}\) to \(\ce{Cr(VI)}\): \[\ce{2Cr(OH)4^{-}(aq) + 3H2O2(aq) + 2OH^{-}(aq) -> 2CrO4^{2-}(aq) + 8H2O(l)}\] To confirm the oxidation, addition of \(\ce{Ba^{2+}}\) solutions precipitate the yellow chromate ion, \(\ce{CrO4^{2-}}\), as yellow barium chromate. \(\ce{Cl^{-}}\), \(\ce{SO4^{2-}}\)
Aqueous Ammonia
Sodium Hydroxide


Hydrogen Peroxide

No Reaction

